 Demographic analysis of fish populations rely on age-structured information.Within age and growth studies,
research on the growth and composition of fish otoliths has enabled researchers to reconstruct the responses
of fishes to their physical and biological environments. It is well known that the microstructure of otoliths
contains lamellar structures that accurately record yearly and daily growth.
Demographic analysis of fish populations rely on age-structured information.Within age and growth studies,
research on the growth and composition of fish otoliths has enabled researchers to reconstruct the responses
of fishes to their physical and biological environments. It is well known that the microstructure of otoliths
contains lamellar structures that accurately record yearly and daily growth.Growth rings in scales, otoliths, opercular bones, fin rays and vertebrae have been used to age fish for a long time. The finding of Pannella in the early 1970's that many teleost fish deposit otolith growth increments with a 24h periodicity, was a major step forward in assessing age and growth with greater accuracy and precision. Deposition of daily increments appears to be a universal phenomenon under all but the most severe conditions. The field of otolith microstructure research is now an accepted, and in most cases, a preferred tool for the study of fish.
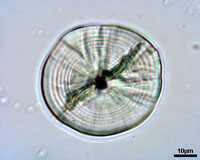
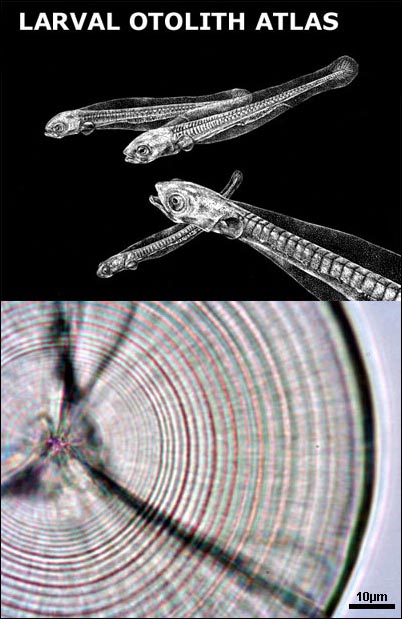
Otoliths serve as a permanent record of the life history of an individual fish. For most species the formation of daily growth rings starts at the end of the yolk sac stage or at the time eyes become pigmented. The exact time of formation of the first increment varies among species. After the first ring around the nucleus concentric rings are formed, and in most cases are believed to be daily. The distance between the rings in influenced by food uptake, temperature and other environmental conditions. The distance between the rings expresses the daily growth of the individual, while the number of rings indicates its age in days.
An image analysis system can be a powerful tool as far as otolith studies are concerned. Image analysis is a generic term used to refer to the digitations and manipulation of visual images. In its simplest form, an image analysis system can store a picture in memory and reproduce it unaltered upon command. In practice images entered into an image analysis system are enhanced and/or quantified before re-display. The end product is an image (or data), which can be more easily interpreted than the original. Image analysis systems should not be confused with video-microscope display units. While the latter have been used to advantage in studies requiring precise otolith measurements, such units are capable of neither image enhancement nor image manipulation. Recent technological advances made microscope-based image analysis systems available to the individual researcher. The majority of these systems consist of a video or a digital camera, a digitizer board mounted within a microcomputer, and a monitor. The video or digital camera should be mounted on a microscope for otolith-based research. The basis of operation for all image analysis systems is the conversion of an image into an array of numbers (image digitization). In the context of microscopic observations, image analysis systems provide three major advantages: image enhancement, manipulation and quantification. Image enhancement is one of the most important and widely used features. Simple procedures allow the operator to subtract and image background from the entire image, average several noisy images, or use high or low frequency filters to add or remove detail. All these enhancement procedures are useful because of the limited capability of the human eye (differentiation of 128 gray levels are beyond visual capabilities). Increase ease of visual interpretation is the primary advantage of an image analysis system in counting growth increments. When measurements are being made, a variety of other features become evident. Despite the undeniable benefits of an image analysis system to otolith microstructure examination, it is important to recognize their limitations. Use of such systems does not improve resolution (while visual contrast can be enhanced, the resolution limit of light microscopy remais the same, around 0,20µm). Secondly, the automatic-count capabilities of many systems are not yet appropriate for studies of otolith microstructure. Finally, image storage presently requires a considerable amount of memory and the creation of large image archives. Image analysis systems are now routine in many scientific disciplines, and their applications to otolith examinations are many-fold. Some of the most frequent applications are as follows: 1) Image enhancement through histogram expansion, filters (convolutions) and edge detection algorithms.2) Image quantification. This broad category includes semi-automated and automated counts and measurements of structures visible in the image. Calibrated measurements of growth increments and otolith size are our most common form of measurement.
3) Shape analysis. This procedure is almost fully automated, and includes measurements of otolith length and area as well as conversion of the outline to Fourier components.
4) Image storage. Subsamples of otolith cross sections are routinely imaged, enhanced and stored to add to existing reference collections for quality control. How to mount a larval otolith (a setp by step guide)
 |
Engraulis encrasicolus larval otolith |
 |
Sardina pilchardus larval otolith |
 |
Sprattus sprattus larval otolith |
-
(i) age determination;
(ii) daily growth rate estimations - population (integrated) and individual growth;
(iii) mortality and recruitment;
(iv) migratory and environmental history;
(v) life history events;
(vi) condition;
(vii) taxonomy and stock structure
2. RÉ, P. (1983). Growth of pilchard larvae, Sardina pilchardus (Walbaum, 1792) in relation to some environmental factors. Investigación Pesquera, 47 (2): 277-283. (IJ)
3. RÉ, P. (1984). Evidence of daily and hourly growth in pilchard larvae based on otolith growth increments, Sardina pilchardus (Walbaum, 1792). Cybium, 8 (1): 33-38. (IJ)
4. ROSA, H. C. & P. RÉ (1985). Influence of exogenous factors on the formation of daily microgrowth increments in otoliths of Tilapia mariae (Boulenger, 1899) juveniles. Cybium, 9 (4): 341-357. (IJ)
5. RÉ, P., H. C. ROSA & M. T. DINIS, (1985). Diel rhythms in Dicentrarchus labrax (L., 1758) larvae under controlled conditions: swim bladder inflation, feeding and otolith growth. Investigación Pesquera, 49 (3): 411-418. (IJ)
6. RÉ, P., H. C. ROSA & M. T. DINIS (1986). Daily microgrowth increments in the sagittae of Dicentrarchus labrax (L.) larvae under controlled conditions. Investigación Pesquera, 50 (3): 397-402. (IJ)
7. RÉ, P. (1986). Otolith microstructure and the detection of life history events in sardine and anchovy larvae. Ciência Biológica. Ecology Sistematics, 6 (1/2): 9-17. (NJ)
8. RÉ, P. (1987). Daily microgrowth increments in fish otoliths. Prospectives in Aquaculture. Cuadernos Marisqueros. Publicación Técnica, 12: 257-260. (CR)
9. RÉ, P., H. C. ROSA & M. T. DINIS (1988). Daily microgrowth increments in otoliths of Solea senegalensis Kaup larvae reared in the laboratory. Ciência Biológica. Ecology Systematics, 8 (1/2): 37-41. (NJ)
10. RÉ, P. (1991). Anéis diários de crescimento nos otólitos dos estados larvares dos peixes: prospectivas em planctonologia. Revista de Biologia da Universidade de Aveiro, Actas do 1º Encontro de Planctonologistas Portugueses: 253-262. (CR)
11. RÉ, P. & E. GONÇALVES (1993). Growth of sprat Sprattus sprattus larvae in the German Bight (North Sea) as inferred from otolith microstructure. Marine Ecology Progress Series, 96: 139-145. (IJ)
12. RÉ, P. & E. GONÇALVES (1993). Growth of sprat (Sprattus sprattus) larvae at stratified and mixed sites in the German bight (North Sea). Boletim UCA, Universidade do Algarve, 2º Encontro de Planctonologistas Portugueses, 1: 269-284. (NJ)
13. RÉ, P. (1993). Estudo micro-estrutural de otólitos de estados larvares de peixes recorrendo ao processamento digital de imagem. Boletim UCA, Universidade do Algarve, 2º Encontro de Planctonologistas Portugueses, 1: 637-652. (NJ)
14. RÉ, P. & L. NARCISO. (1994). Growth and cuttlebone microstructure of juvenile cuttlefish, Sepia officinalis L., under controlled conditions. Journal of Experimental Marine Biology and Ecology, 177: 73-78. (IJ)
15. HAKANSON, J.L., S.H. COOMBS & P. RÉ (1994). Lipid and elemental composition of sprat (Sprattus sprattus) larvae at mixed and stratified sites in the German Bight of the North Sea. ICES Journal of Marine Sciences, 51: 147-154. (IJ)
16. RÉ, P. (1994). Anéis diários de crescimento nos otólitos dos estados larvares dos peixes: prospectivas em biologia pesqueira. Professor Germano da Fonseca Sacarrão, Museu Bocage, Lisboa: 97-124. (CB)
17. RÉ, P. (1996). Anchovy spawning in the Mira Estuary (soutwestern Portugal). Scientia Marina, The European Anchovy and its Environment I.Palomera and P.Rubiés (eds), 60 (Supl. 2): 141-153. (CB)
18. CHÍCHARO, M.A., L. CHÍCHARO, L. VALDEZ, E. LOPEZ-JAMAR & P. RÉ (1998). Estimation of starvation and diel variation of the RNA/DNA ratios in field-caught Sardina pilchardus (L.) larvae off the north of Spain. Marine Ecology Progress Series, 164: 273-283. (IJ)
19. CHÍCHARO, M.A., L. CHÍCHARO, E. LOPEZ-JAMAR, L. VALDEZ & P. RÉ (1998). Does the nutritional condition represent a severelimit to the survival of the sardine (Sardina pilchardus) larvae from north coast of Spain? Results based on RNA/DNA ratios and their variability. Fisheries Research, 39: 43-54. (IJ)
20. RÉ, P. & R. KNUST, 1996 (2001). Daily growth of sprat larvae (Sprattus sprattus) in the German Bight of the North Sea during the 1991 spawning season. Ciência Biológia. Ecology and Systematics (Portugal), 16 (1/2): 209-225. (NJ)
21. CHÍCHARO, M.A., E. ESTEVES, A.M.P. SANTOS, A. DOS SANTOS, A. PELIZ, P. RÉ (2003). Are sardine larvae caught off northern Portugal in winter starving? An approach examining nutritional conditions. Marine Ecology Progress Series, 257: 303-309. (IJ)
22. SANTOS, A.M.P., A. PELIZ, J. DUBERT, P.B. OLIVEIRA, M.M. ANGÉLICO, P. RÉ (2004). Impact of a winter upwelling event on the distribution and transport of sardine eggs and larvae off western Iberia: A retention mechanism. Continental Shelf Research, 24: 149-165. (IJ)
23. SANTOS, A.M.P., P. RÉ, A. dos SANTOS, A. PELIZ (2006). Vertical distribution of the European sardine (Sardina pilchardus) larvae and its implications for their survival. Journal of Plankton Reasearch, 28 (5): 523-532. doi:10.1093/plankt/fbi137 (IJ)
24. SANTOS, A.M.P., A. CHÍCHARO, A. DOS SANTOS, T. MOITA, P.B. OLIVEIRA, A. PELIZ, P. RÉ (2007). Physical-biological interactions in the life history of small pelagic fish in the Western Iberia Upwelling Ecosystem. Progress in Oceanography. 74: 192-209 (IF- 2,432) (IJ)
25. GARRIDO, S., E. SAIZ, J. PETERS, P. RÉ, P. ALVAREZ, U. COTANO, D.L. HERRERO, A. MARTÍNEZ DE MURGUÍA , X. IRIGOIEN (2012). Effect of food type and concentration on growth and fatty acid composition of early larvae of the anchovy (Engraulis encrasicolus) reared under laboratory conditions. Journal of Experimental Marine Biology and Ecology 434-435 (2012) 16-24 http://dx.doi.org/10.1016/j.jembe.2012.07.021 (IJ) Otolith Research Laboratory - Guia Marine Laboratory

Otolith Research Laboratory (2003/2004)
Otolith Research Laboratory (2005)
Otolith Research Laboratory (2012)
-
- Two Pentium-based PCs
- Two 19" monitors
- Olympus DP10 & Leica EC3 cameras
- Dissecting and compound microscopes (Wild M5A, Leica S6D and Olympus BH-2)
- B&W laser and colour printers
- Colour scanner
- Image Pro Plus Software
- Two Pentium-based PCs
-
- Larval and juvenile otolith microstructure studies (mainly Clupeoids).
- Recruitment and mortality studies
- Age validation studies
- Photographic atlas of larval fish otolith
- Larval and juvenile otolith microstructure studies (mainly Clupeoids).

 CLUPEIDAE
CLUPEIDAE
ENGRAULIDAE
ARGENTINIDAE
STERNOPTYCHIDAE
MYCTOPHIDAE
PARALEPIDIDAE
GADIDAE
CARAPIDAE
LOPHIIDAE
BELONIDAE
SERRANIDAE
CEPOLIDAE
CARANGIDAE
AMMODYTIDAE
SCOMBRIDAE
GOBIIDAE
BLENNIIDAE
MUGILIDAE
CALLIONYMIDAE
SCORPAENIDAE
TRIGLIDAE
BOTHIDAE
SOLEIDAE